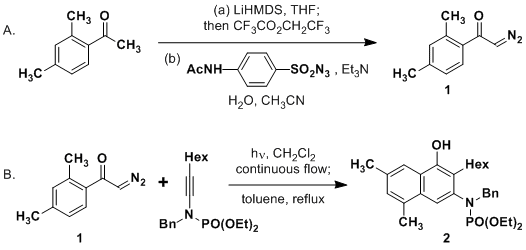
Scheme 1. Synthesis of protected aldehydes of 3,5-dimethylthiotetronic... | Download Scientific Diagram

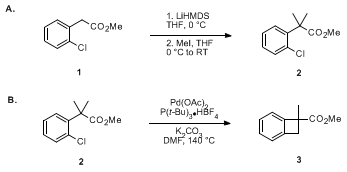
Efficient one-pot selective reduction of esters in β-ketoesters using LiHMDS and lithium aluminium hydride - ScienceDirect

LiHMDS: Facile, highly efficient and metal-free transesterification under solvent-free condition - ScienceDirect
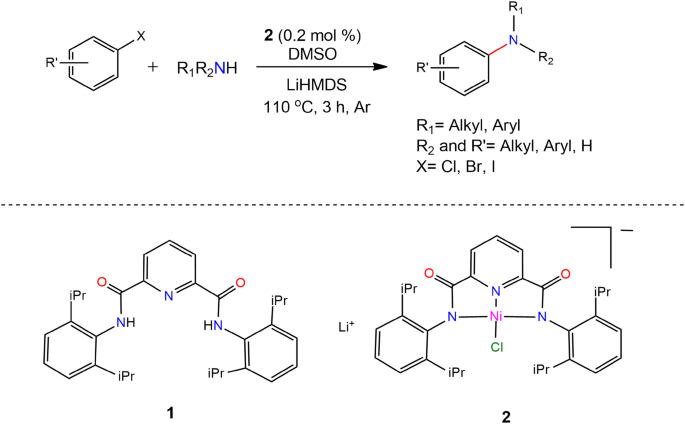
C–N Cross-coupling Reactions of Amines with Aryl Halides Using Amide-Based Pincer Nickel(II) Catalyst | SpringerLink

LiHMDS: Facile, highly efficient and metal-free transesterification under solvent-free condition - ScienceDirect
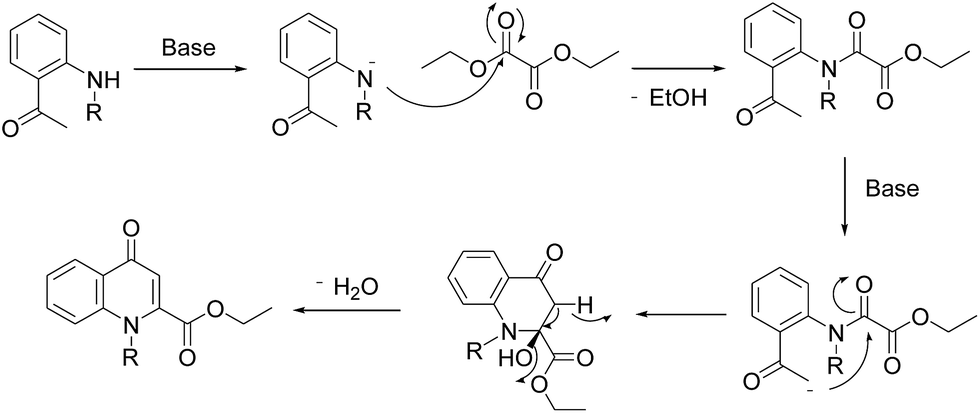
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C
Lithium Hexamethyldisilazide-Mediated Enolization of Acylated Oxazolidinones: Solvent, Cosolvent, and Isotope Effects on Competi
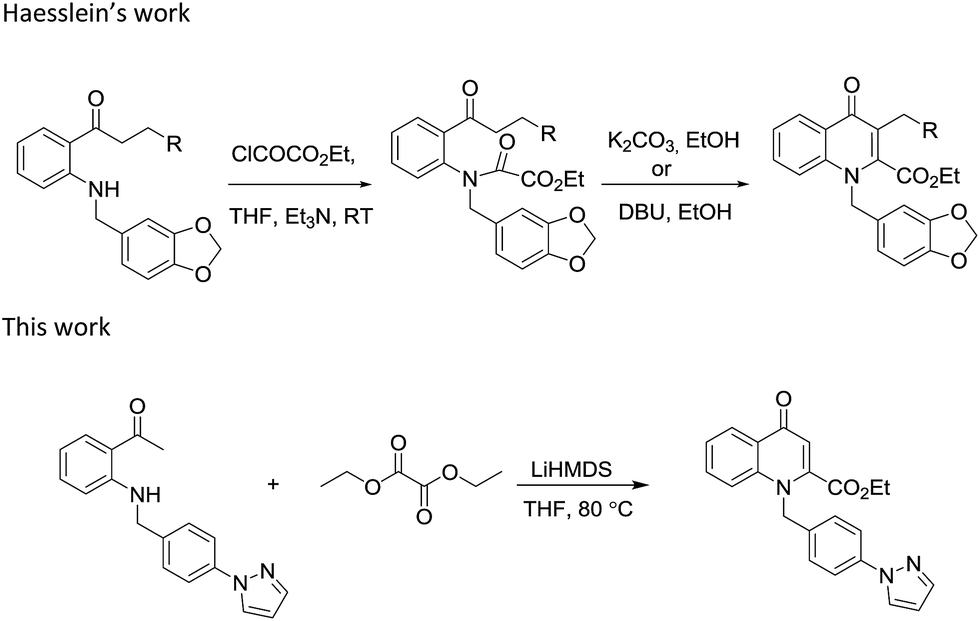
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C
Mechanism and Scope of Baseâ•'Controlled Catalystâ•'Free Nâ•'Arylation of Amines with Unactivated Fluorobenzenes
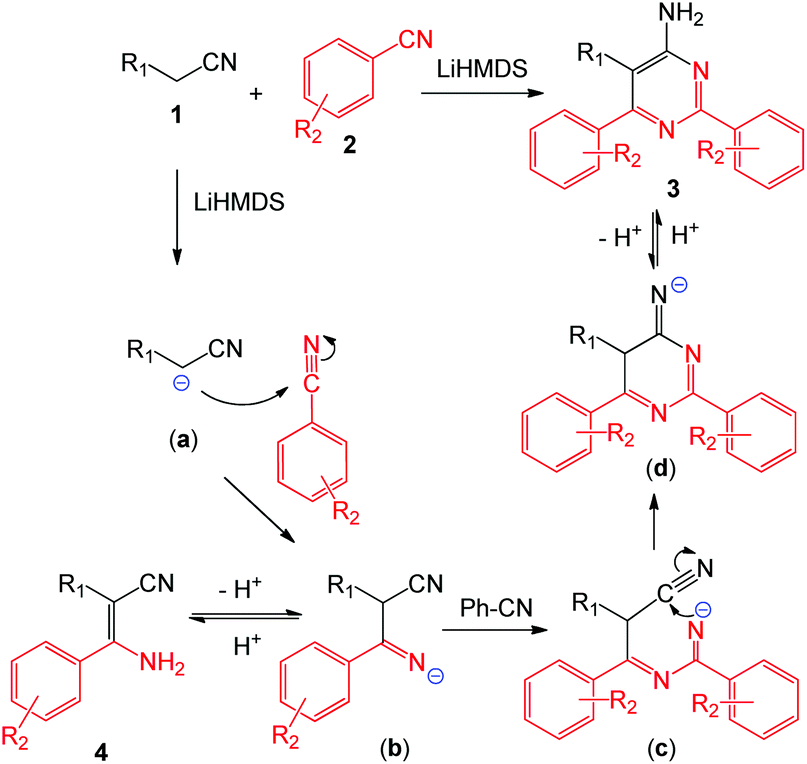
Utilization of nitriles as the nitrogen source: practical and economical construction of 4-aminopyrimidine and β-enaminonitrile skeletons - Organic Chemistry Frontiers (RSC Publishing)
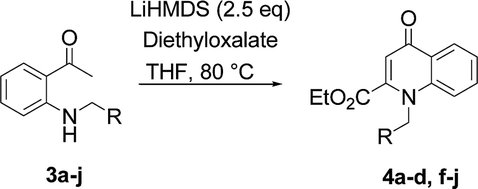
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C
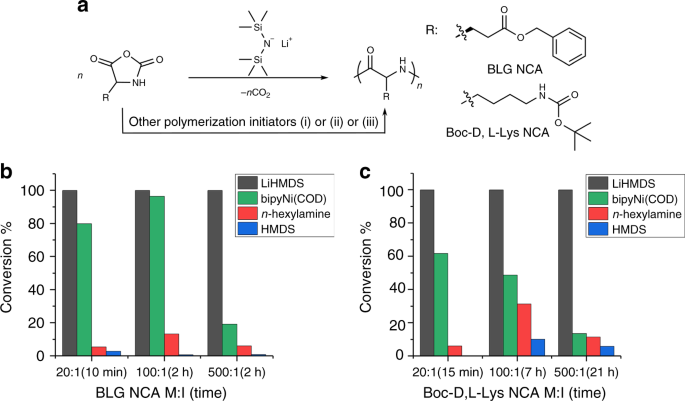
Lithium hexamethyldisilazide initiated superfast ring opening polymerization of alpha-amino acid N -carboxyanhydrides | Nature Communications

Scheme 1. Synthesis of inactivator 3.a )2,2-dimethyl-1,3-dioxan-5-one,... | Download Scientific Diagram

Lithium hexamethyldisilazide-mediated enolizations: influence of triethylamine on E/Z selectivities and enolate reactivities. - Abstract - Europe PMC
Lithium Hexamethyldisilazide-Mediated Enolization of Acylated Oxazolidinones: Solvent, Cosolvent, and Isotope Effects on Competi
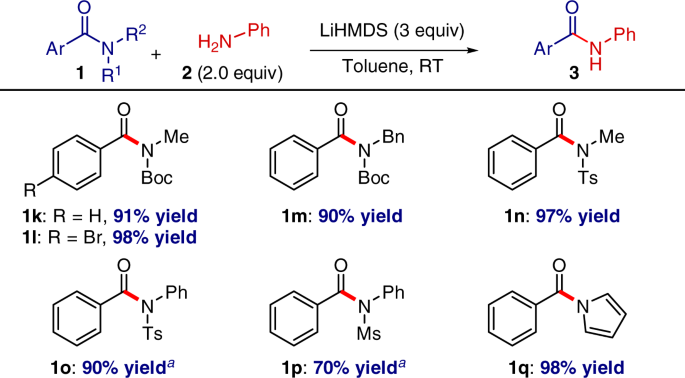
Highly selective transition-metal-free transamidation of amides and amidation of esters at room temperature | Nature Communications