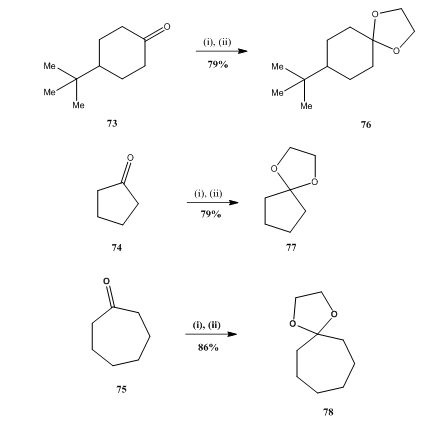
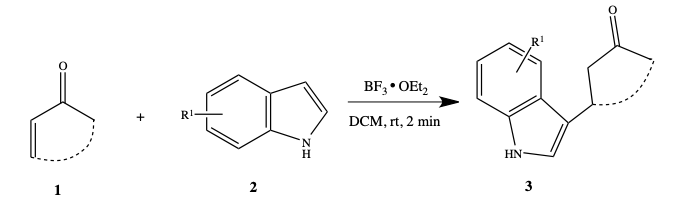
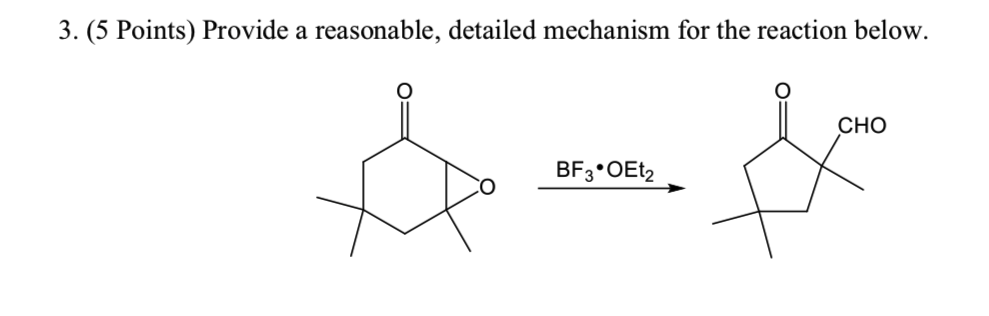
BF3·OEt2 Mediated Regioselective Reaction of Electron-Rich Arenes with 3-Ylidene Oxindoles. | Semantic Scholar
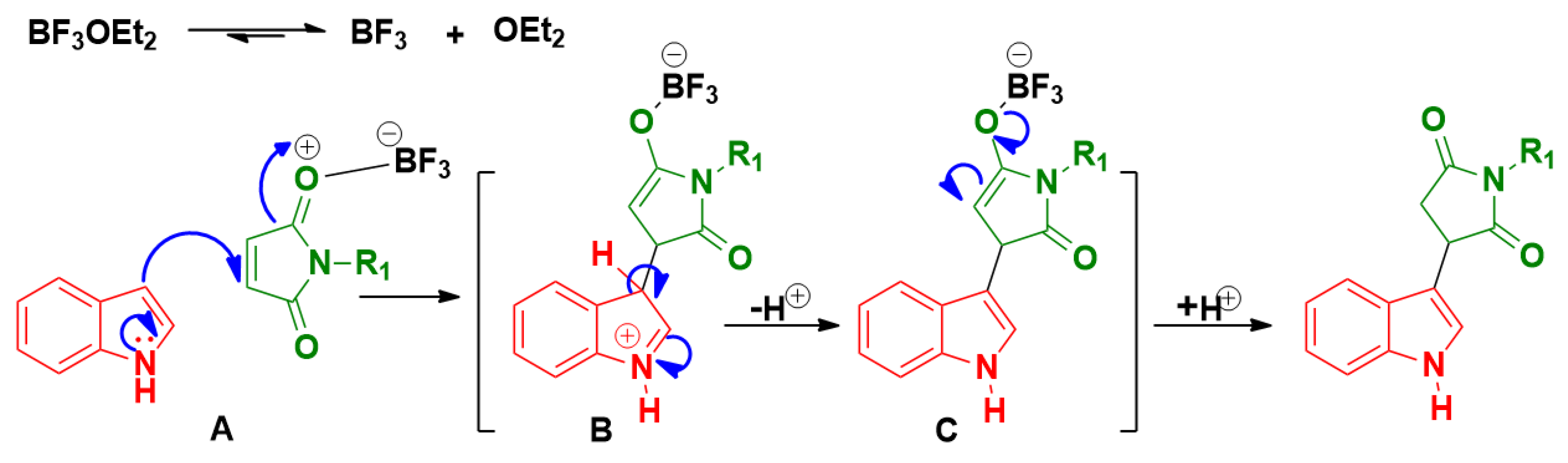
Molecules | Free Full-Text | BF3-OEt2 Catalyzed C3-Alkylation of Indole: Synthesis of Indolylsuccinimidesand Their Cytotoxicity Studies
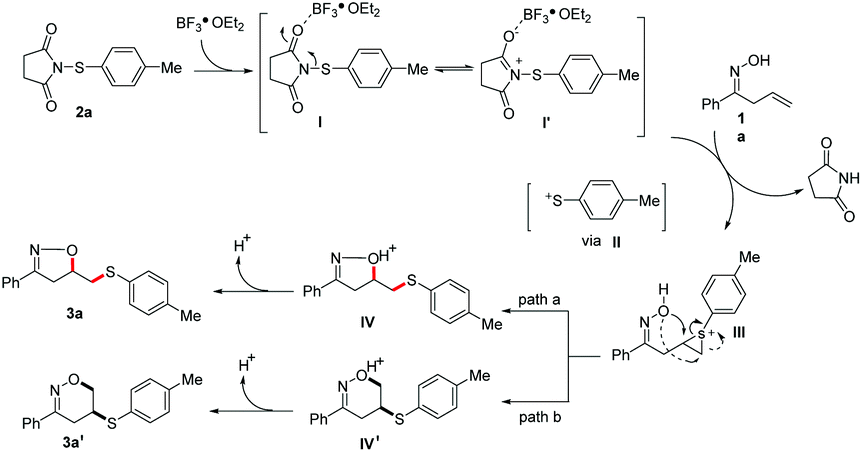
BF 3 ·OEt 2 -mediated cyclization of β,γ-unsaturated oximes and hydrazones with N -(arylthio/arylseleno)succinimides: an efficient approach to synthes ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D0OB01388A

Lewis acid BF3·OEt2-catalyzed Friedel–Crafts reaction of methylenecyclopropanes with arenes - ScienceDirect

Glycosyl Fluorides as Intermediates in BF3·OEt2‐Promoted Glycosylation with Trichloroacetimidates - Nielsen - 2017 - European Journal of Organic Chemistry - Wiley Online Library

Revisit of the phenol O-glycosylation with glycosyl imidates, BF3·OEt2 is a better catalyst than TMSOTf - ScienceDirect

Glycosyl Fluorides as Intermediates in BF3·OEt2‐Promoted Glycosylation with Trichloroacetimidates - Nielsen - 2017 - European Journal of Organic Chemistry - Wiley Online Library

Regioselective ring-opening of epoxides with ortho-lithioanisoles catalyzed by BF3·OEt2 - ScienceDirect
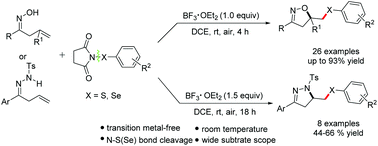
BF3·OEt2-mediated cyclization of β,γ-unsaturated oximes and hydrazones with N-(arylthio/arylseleno)succinimides: an efficient approach to synthesize isoxazoles or dihydropyrazoles - Organic & Biomolecular Chemistry (RSC Publishing)
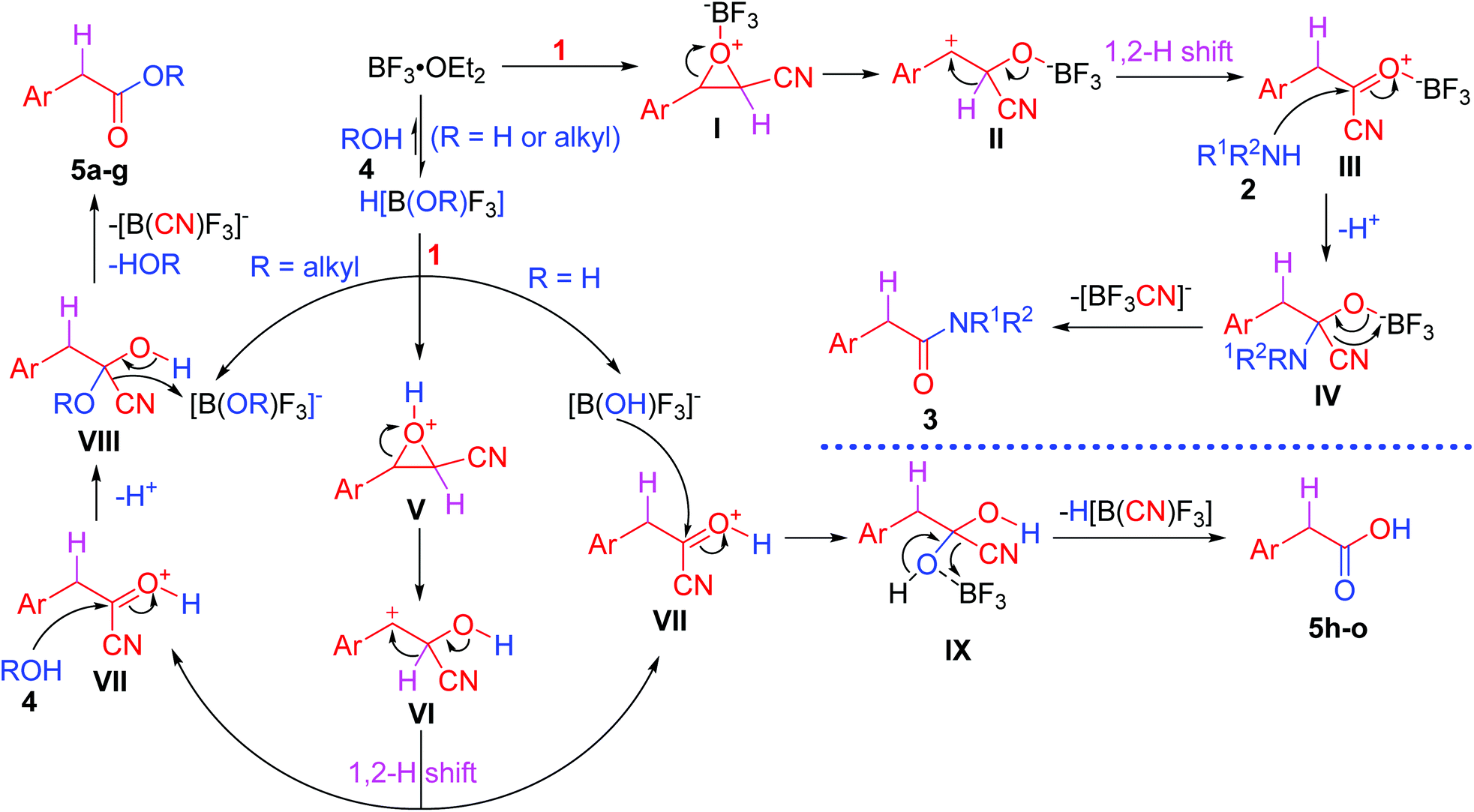
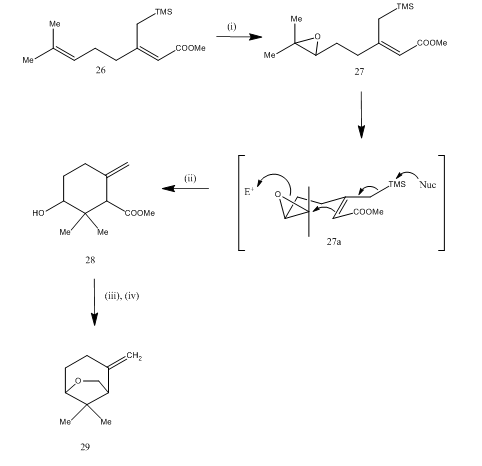
BF 3 ·OEt 2 -promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB02428J
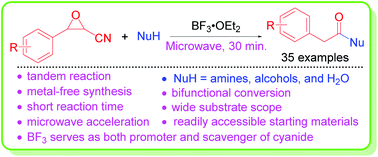
BF3·OEt2-promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles - Organic & Biomolecular Chemistry (RSC Publishing)

BF3·OEt2-promoted concise synthesis of difluoroboron-derivatized curcumins from aldehydes and 2,4-pentanedione - ScienceDirect

An expeditious method to synthesize difluoroboron complexes of β-keto amides from β-keto nitriles and BF3·OEt2 | Request PDF

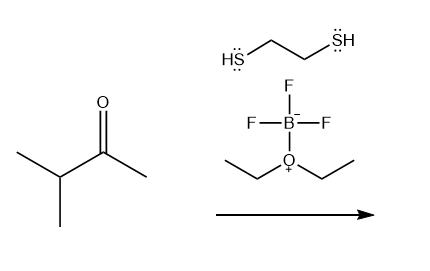
BF3 ⋅ OEt2 Catalyzed Synthesis of 1,3‐Thiazines/‐Selenazines - Muthusamy - 2021 - Asian Journal of Organic Chemistry - Wiley Online Library